“It is an inherited disease characterized by hemolytic anemia caused by inability to detoxify oxidized agents”.
- Inherited X-linked recessive disease.
- Most common enzyme-related hemolytic anemia.
- Highest prevalence: Middle East, Tropical Africa, Asia, and Mediterranean.
Mode of inheritance
- It is X-linked recessive genetic disorder (gene is carried on X chromosome).
- The inheritance follows specific pattern:
-
Males have one X chromosome, so they will be diseased if they have the affected gene (xY).
-
Females have 2 X chromosomes, may be homozygous or heterozygous.
-
Homozygous: are diseased (xx).
-
Heterozygous: are not diseased BUT: carriers (Xx) & can transfer the disease to their sons.
-
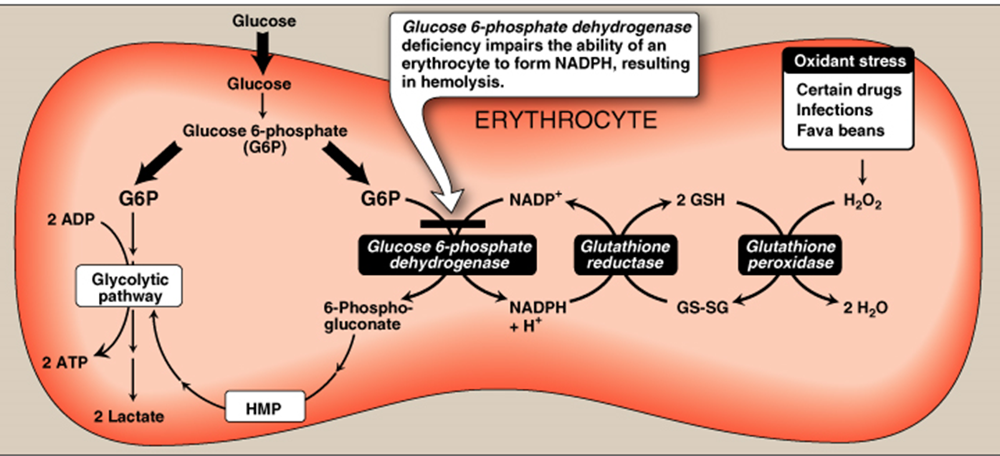
The function G6PD in the red cell
- To generate NADPH → reduced glutathione → protect the RBCs from the oxidative damage by H2O2.
- Reduction of amounts of NADPH in RBCs in G6PD deficiency causes decrease in reduction of oxidized glutathione to reduced glutathione.
Role of reduced glutathione in RBCs:
- Reduced Glutathione gets rid of ROS including hydrogen peroxide.
- Reduced Glutathione helps to keep sulfhydryl groups of hemoglobin protein in the reduced state.
Reduction of production of reduced glutathione results in:
-
A decrease in detoxication of peroxides. This causes damage to RBCs membrane and hemolysis (ending in hemolytic anemia).
-
Hemoglobin protein is denatured forming insoluble masses (Heinz bodies). Heinz bodies attach to red cell membranes. Membrane proteins are also oxidized. Accordingly, red cells become rigid and removed from the circulation by macrophages in the spleen and liver ending in anemia.
Deficiency of G6PD occurs in all cells of affected individual. It is severe in RBCs because the only pathway to form NADPH in RBCs is pentose phosphate pathway (using G6PD).
Biochemical Basis of G6PD Deficiency Hemolytic Anemia
Decreased amounts of reduced glutathione due to decreased production of NADPH.
In G6PD deficiency
-
In G6PD deficiency; younger RBC contain 6% of the normal amount of enzyme, sufficient to provide reducing power for every day needs.
-
G6PD deficiency usually does not cause any symptoms unless the patient takes one of a number of drugs, such as the anti-malarial primaquine. The administration of one of these drugs causes rapid and often severe hemolysis and jaundice.
Clinical Features:
- Acute hemolysis: red blood cells no longer transport oxygen effectively.
- Drugs, infections, diabetic acidosis.
- Favism: Some G6PD deficient individuals are allergic to fava beans and the smell of the fava pollen.
- Neonatal jaundice: Jaundice most often appears within the first 24 hours of life in (Class I variants). Some G6PD-deficient neonates, if undiagnosed soon after birth, could present later in the first week of life with generalized jaundice, poor feeding, lethargy, breathing difficulty, or seizures. Can lead to kernicterus. Class II or III.
Other conditions caused by G6PD deficiency:
-
Chronic nonspherocytic hemolytic anemia: male, history of neonatal jaundice present with anemia, unexplained jaundice, or gallstones later in life, severe deficiency (eg, activity <10 percent at baseline). Neutrophil dysfunction.
-
Abdominal & back pain, dizziness, irregular breathing, and palpitations.
-
G6PD deficient patients have increased resistance to infestation by falciparum malaria.
---x
| Category | Definitive Risk | Possible Risk | Doubtful Risk |
|---|---|---|---|
| Antimalarials | Primaquine, Dapsone, Chlorproguanil | Chloroquine | Quinine |
| Sulphonamides/Sulphones | Sulphamethoxazole, Dapsone | Sulfasalazine, Sulfadimidine | Sulfisoxazole, Sulfadiazine |
| Antibacterials/Antibiotics | Cotrimoxazole, Nalidixic acid, Nitrofurantoin | Ciprofloxacin, Norfloxacin | Chloramphenicol, p-Aminosalicylic acid |
| Antipyretic/Analgesics | Acetanilide, Phenazopyridine (Pyridium) | Acetylsalicylic acid (High dose > 3g/d) | Acetylsalicylic acid (< 3g/d), Acetaminophen |
Diagnosis
- Diagnosis of hemolytic anemia: CBC and reticulocytic count.
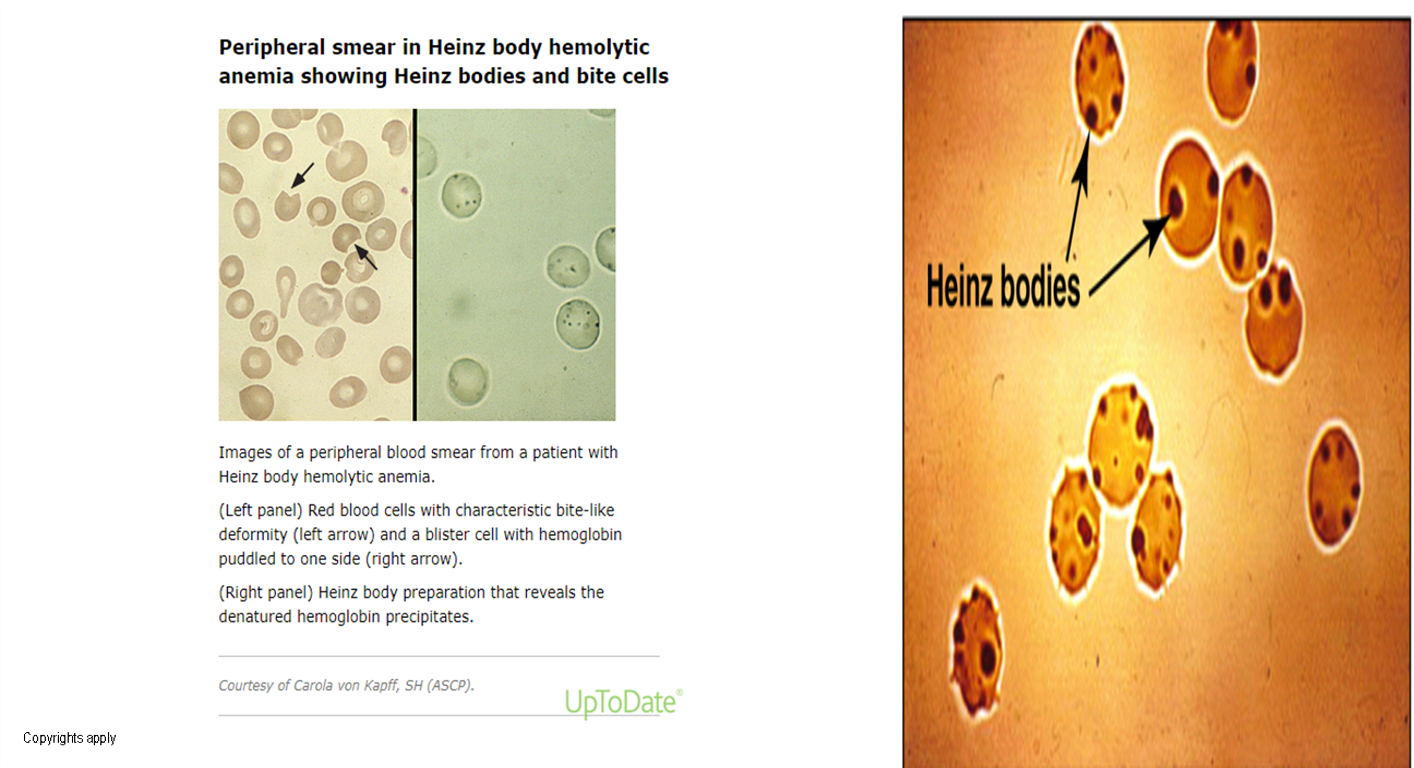
- Peripheral smear: bite cells, Heinz bodies (Oxidation of sulfhydryl groups of proteins inside RBCs causes protein denaturation and formation of insoluble masses), and polychromasia.
- Screening: Qualitative assessment of G6PD enzymatic activity (UV-based test), if initial testing is negative and a suspicion for G6PD deficiency remains, testing should be repeated approximately three months after the hemolytic episode has resolved.
- Confirmatory test: Quantitative measurement of G6PD enzymatic activity.
- Molecular test: Detection of G6PD gene mutation.
A1C values underestimate blood glucose in G6PD deficiency
- Glycated hemoglobin (A1C) estimates average blood glucose over time. When red blood cell turnover increases, as in hemolytic anemias, there is less time for hemoglobin to be glycosylated, and the A1C underestimates glycemia.
Treatment
-
Scientists can genetic engineer fava bean, so that it will no longer react to G6PD individual’s red blood cells.
-
The best treatment now is prevention: G6PD deficient people should not eat fava beans or take drugs that contain oxidizing agents.