Acid Base Disturbance
Prepared by:
Dr. Salma ElGazzar
Objectives
- Recall the physiology involved in the acid-base balance of the body.
- Describe the normal values and factors that influence normal variables.
- Identify abnormal values and their interpretation.
- Describe and list the differential diagnosis of both respiratory acidosis and alkalosis.
- Describe and list the differential diagnosis of both metabolic acidosis and alkalosis.
Introduction
Definitions
Acid
An acid is a substance that can release a hydrogen ion (H+).
Base
A base is a substance that can accept a hydrogen ion (H+).
Buffer
A buffer is a chemical that minimizes the change in pH when an acid or base is added to a solution. The main buffer in the human body is carbonic acid (H2CO3). Carbonic acid buffers blood through the following reaction:
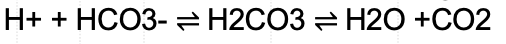
Anions and Cations
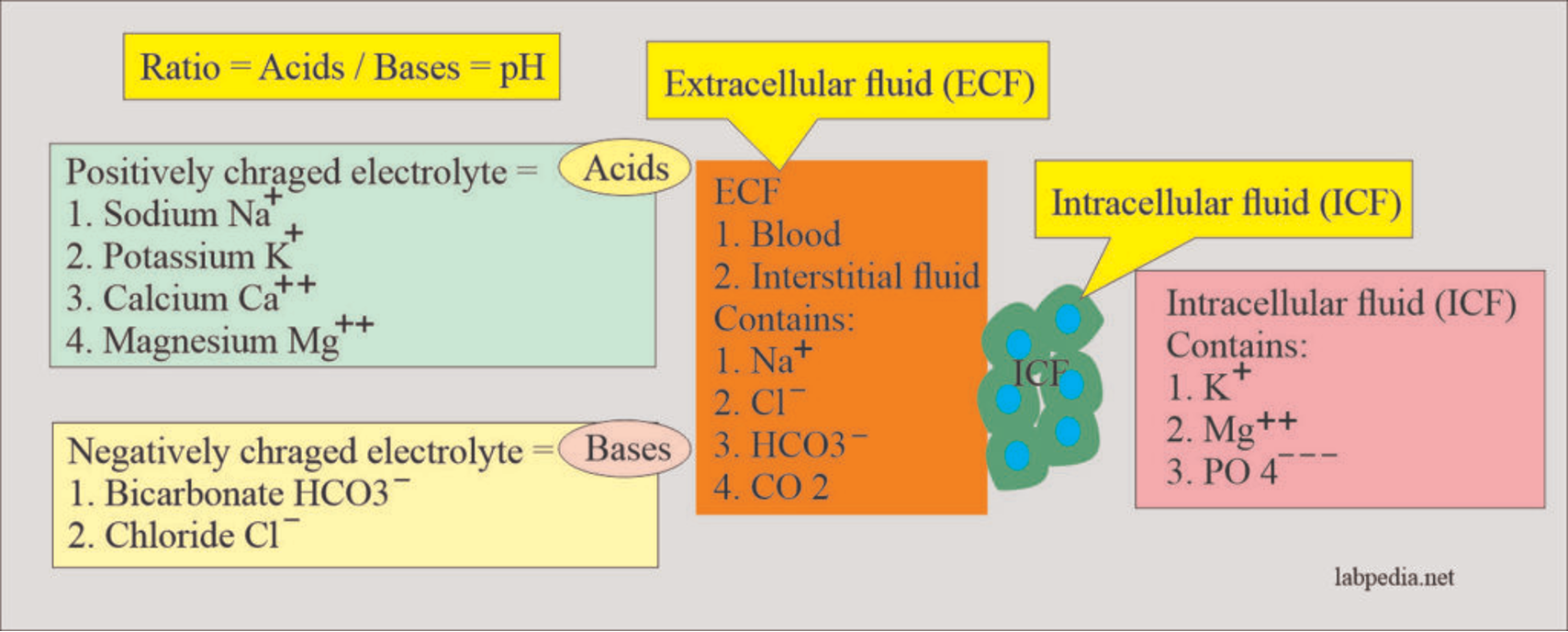
- Anions are atoms or groups of atoms that carry a negative charge. The main anions in human blood are chloride (Cl-) and bicarbonate (HCO3-).
- Cations are atoms or groups of atoms that carry a positive charge. The main cation in human blood is sodium (Na+).
Physiology of the Acid-Base Balance
Acid–base balance is the regulation of the extracellular fluid environment involving the ratio of acid to base, measured clinically as pH. Physiologically, all positively charged ions are called acids, and all negatively charged ions are called bases.
Ratio = Acids / Bases = pH
 https://labpedia.net/acid-base-balance-part-1-introduction-to-the-acid-base-balance/
https://labpedia.net/acid-base-balance-part-1-introduction-to-the-acid-base-balance/
Body Acids
Body acids are formed from end products of:
- Metabolism of proteins.
- Metabolism of carbohydrates.
- Metabolism of fats.
This must be balanced by the number of basic substances in the body to maintain the normal pH. Lungs, kidneys, and bones are the major organs involved in the regulation of acid-base balance.
Types of Body Acids
-
Volatile Acids:
- Carbonic acid (H2CO3) is a weak acid and does not easily release the H ions.
- The presence of carbonic anhydrase enzyme can eliminate CO2 gas and water (H2O).
- CO2 is eliminated through the lungs.
-
Nonvolatile Acids:
- These include sulfuric acid, phosphoric acid, and other organic acids that are eliminated through the kidneys.
- These are strong acids and readily give up their H ions.
- Nonvolatile acids are secreted into the urine by the renal tubules.
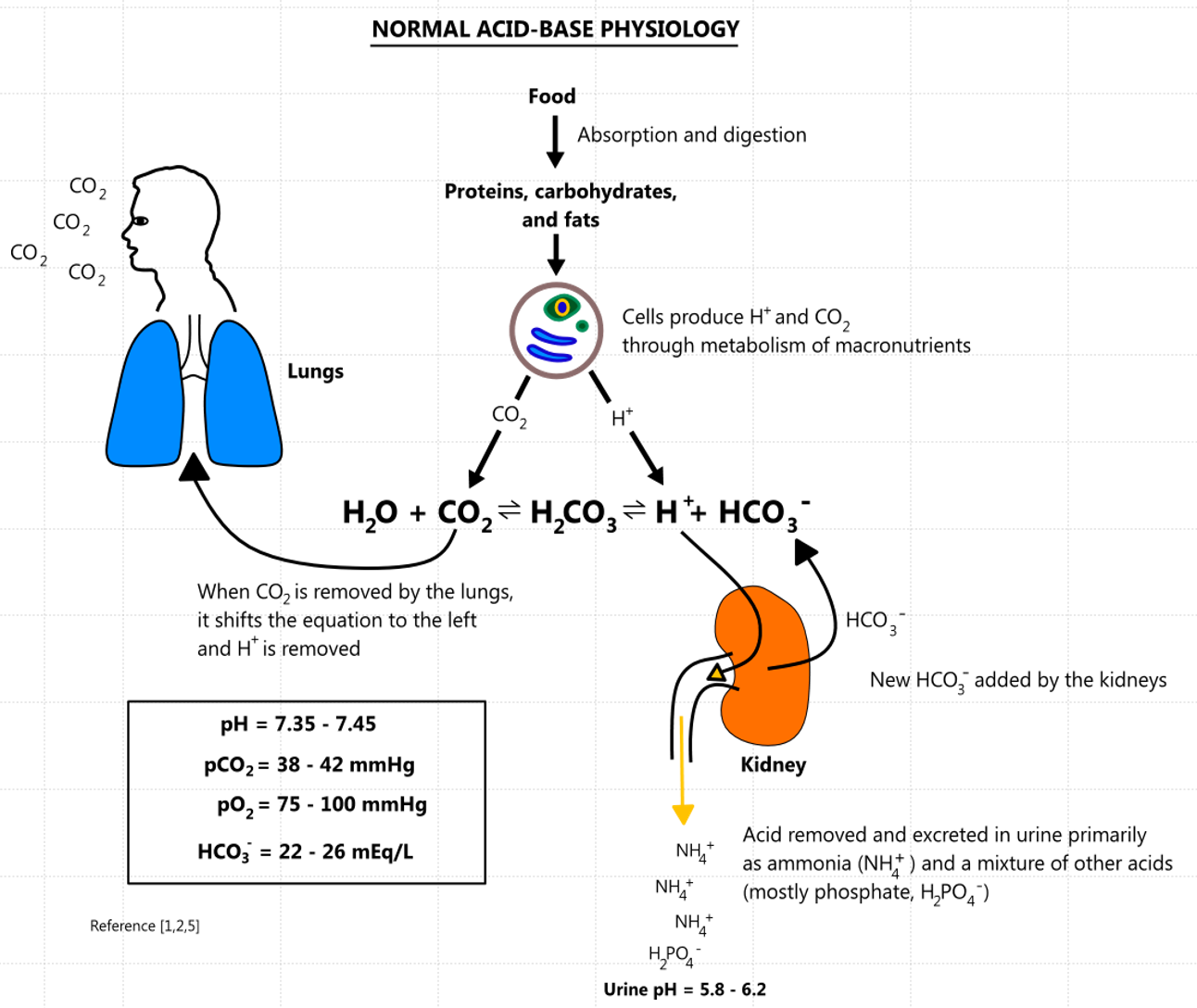
Macronutrient Metabolism
Macronutrient metabolism typically generates a net gain of acid:
-
Carbohydrates and fats are oxidized to CO2 and H2O.
-
The CO2 immediately reacts with H2O in the blood to produce H+ and HCO3- via the following reaction:
[ H_2O + CO_2 \ H_2CO_3 \ H^+ + HCO_3^- ] -
Proteins are metabolized to strong acids (e.g., H2SO4, HCl, H3PO4).
To maintain a normal pH, the body must buffer and eliminate the CO2 and strong acids that are produced. CO2 is typically eliminated through the lungs at the same rate that it is produced. The kidneys help remove strong acids by excreting H+, and they generate new HCO3- to replace HCO3- lost through buffering. Other chemical buffers exist (e.g., proteins, phosphate, hemoglobin, bone minerals, etc.), but carbonic acid (H2CO3) is the most important one for maintaining blood pH.
Acid-Base Homeostasis
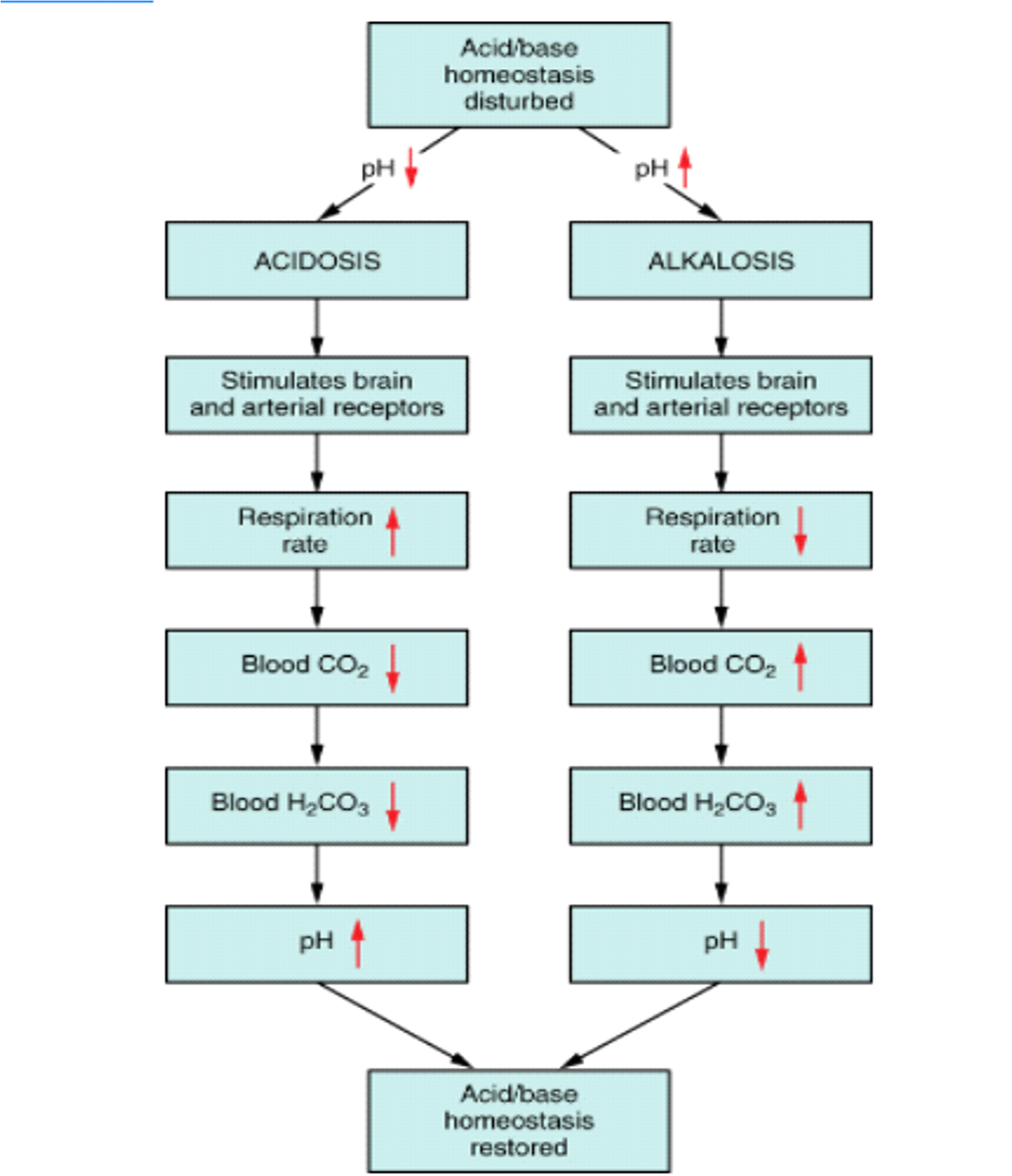
The lungs can raise pH by blowing off more CO2, and they can lower pH by decreasing ventilation and retaining CO2.
Respiratory compensation can begin within minutes and becomes maximal in 12 - 24 hours.
If the primary disorder is from a metabolic cause, the lungs will compensate (respiratory compensation).
Role of Kidneys in Acid-Base Balance
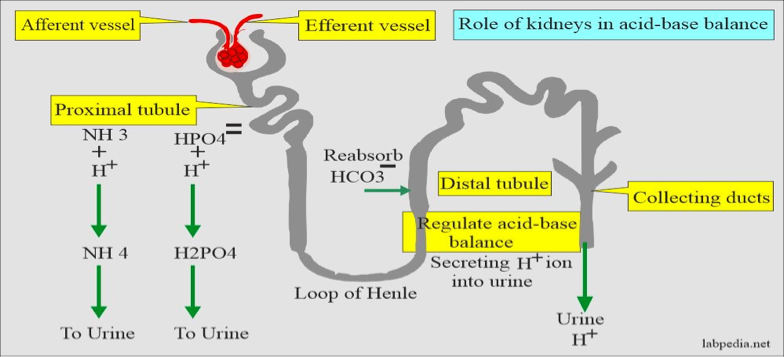 The kidneys can raise pH by increasing the production of HCO3- and excreting acid, and they can lower pH by eliminating HCO3- and retaining acid.
The kidneys can raise pH by increasing the production of HCO3- and excreting acid, and they can lower pH by eliminating HCO3- and retaining acid.
Renal compensation has an acute and chronic phase, where the effect is smaller at first before reaching a peak in 2 - 5 days.
If the primary acid-base disorder is from a respiratory cause, the kidneys will compensate (renal compensation).
Definitions and Normal Value of ABG
Lab Test Definitions
-
pH:
- Increased pH value indicates alkalosis.
- Decreased value of pH indicates acidosis.
-
pCO2:
- This is the partial pressure of CO2, and it will tell:
- The respiration modulates this pCO2.
- This is the index of ventilation.
-
pO2:
- This is the partial pressure of the O2 in the arterial blood and tells:
- Low values indicate hypoxia.
- pO2 is the indirect measure of O2 contents of arterial blood.
-
Base Excess:
- Measures the change in the concentration of a buffer base from the normal value.
- Normal range = +/- 2 mmol/L.
-
Anion Gap:
- ([Na^+ + K^+] - [Cl^- + HCO_3^-]).
- Normal = 10 - 16 mmol/L.
- Reflects the concentration of those anions not routinely measured, e.g., organic acids.
Blood gas measurement can identify the primary disturbance in acid-base balance.
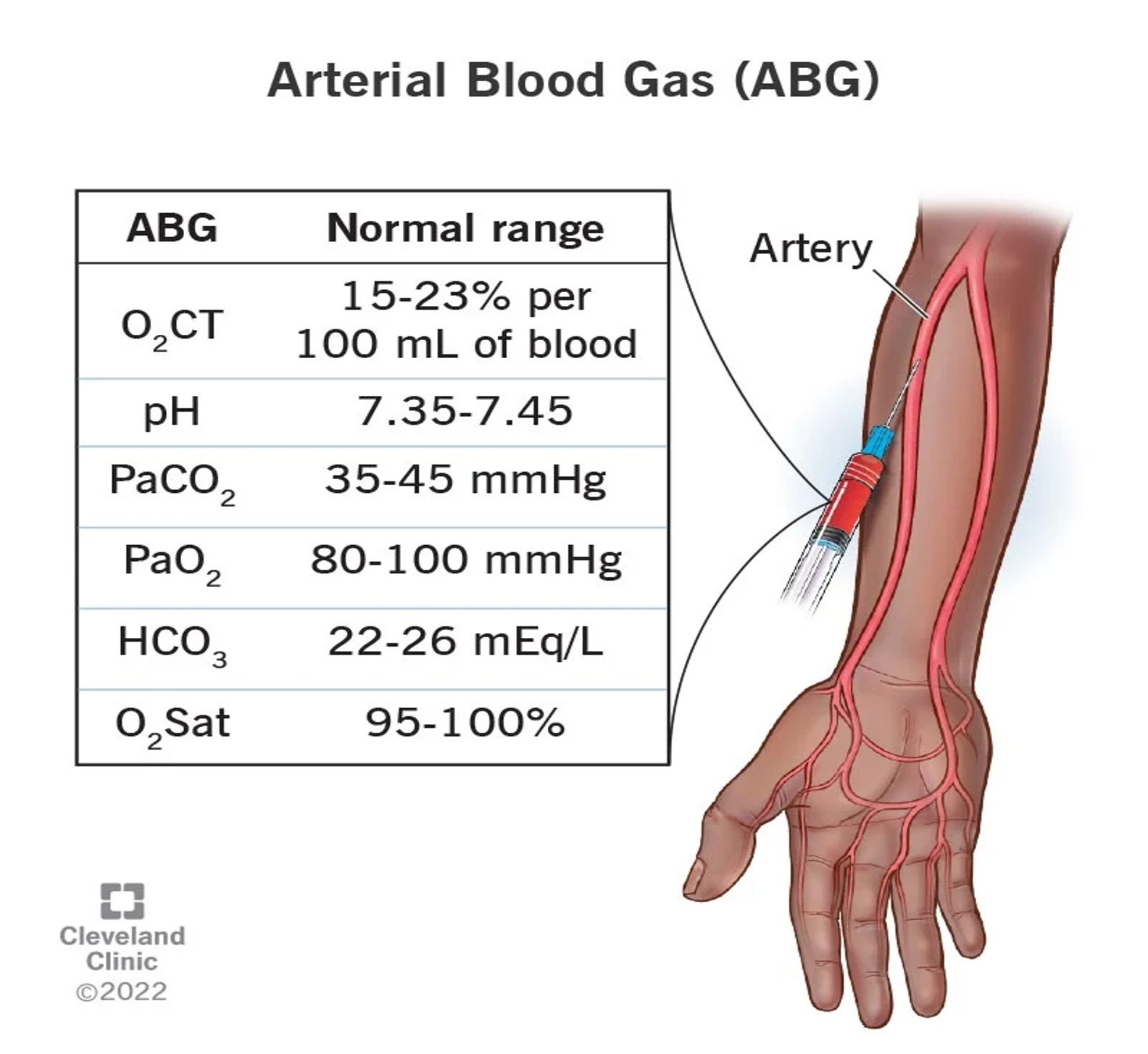
Normal Range of Arterial Blood Gas (ABG)
- O2 CT: 15-23% per 100 ml of blood
- pH: 7.35-7.45
- PaCO2: 35-45 mmHg
- PaO2: 80-100 mmHg
- HCO3: 22-26 mEq/L
- O2 Sat: 95-100%
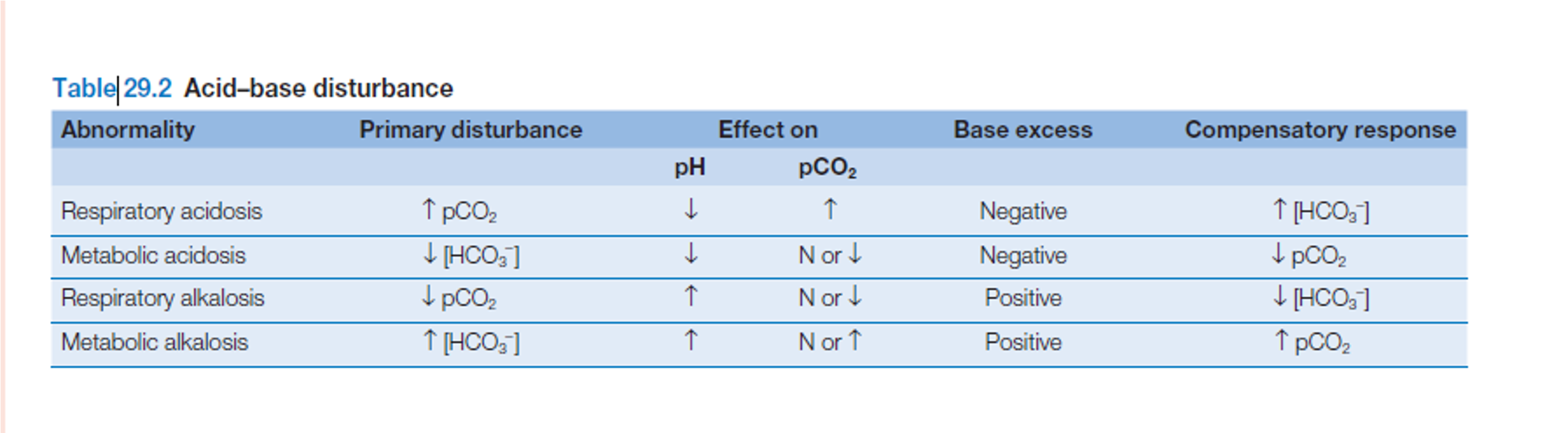
ABG interpretation
For the purpose of this guide, we have set three (3) goals that we need to accomplish when interpreting arterial blood gases. The goals are as follows:
-
Based on the given ABG values, determine if values interpret ACIDOSIS or ALKALOSIS.
-
Second, we need to determine if values define METABOLIC or RESPIRATORY.
-
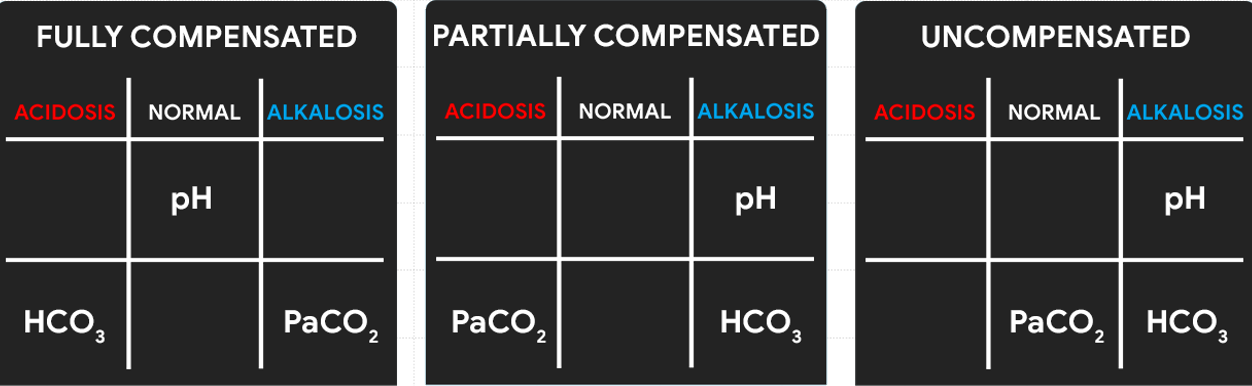
Lastly, we need to determine the compensation if it is: FULLY COMPENSATED, PARTIALLY COMPENSATED, or UNCOMPENSATED.
Normal Blood pH Scale
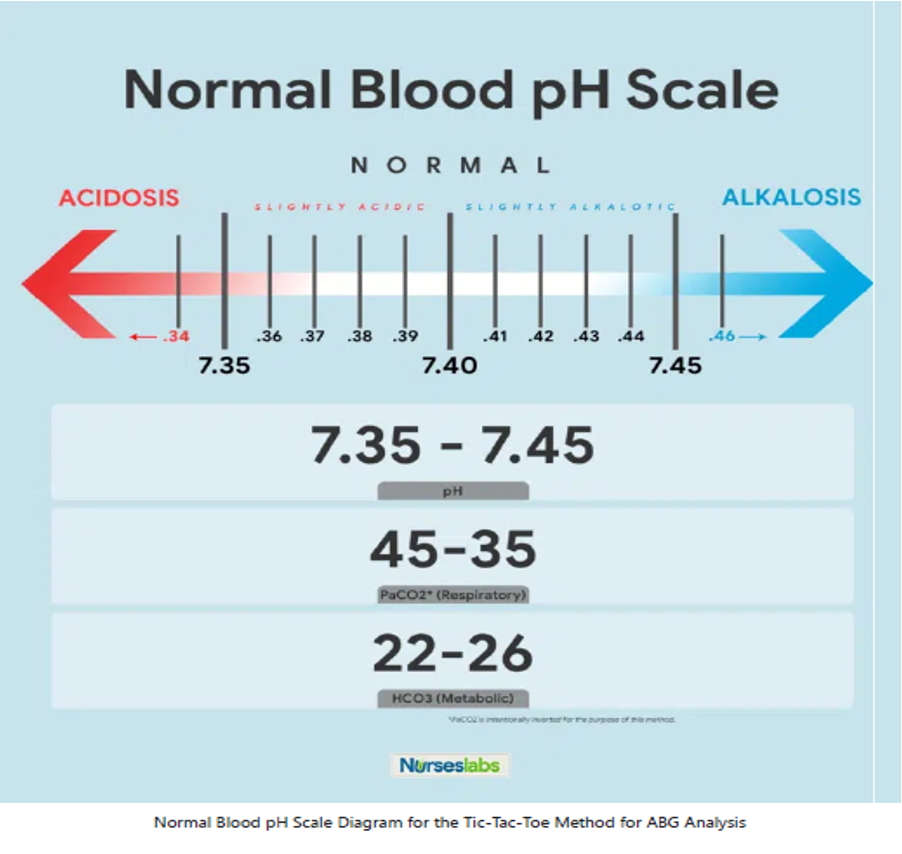
- Normal Range for pH: 7.35 to 7.45
- Normal Range for PaCO2: 35 to 45
- Normal Range for HCO3: 22 to 26
Based on the given ABG values, determine if values interpret ACIDOSIS or ALKALOSIS.
- Make a 3×3 grid and label.
- Based on their values, determine in which column we’ll place pH, PaCO2, and HCO3 in the grid.
- Determine the acidity or alkalinity of the blood with the given value of the pH:
- Any blood pH below 7.35 (7.34, 7.33, 7.32, and so on…) is ACIDOSIS, place it under the ACIDOSIS column.
- Any blood pH above 7.45 (7.46, 7.47, 7.48, and so on…) is ALKALOSIS, place it under the ALKALOSIS column.
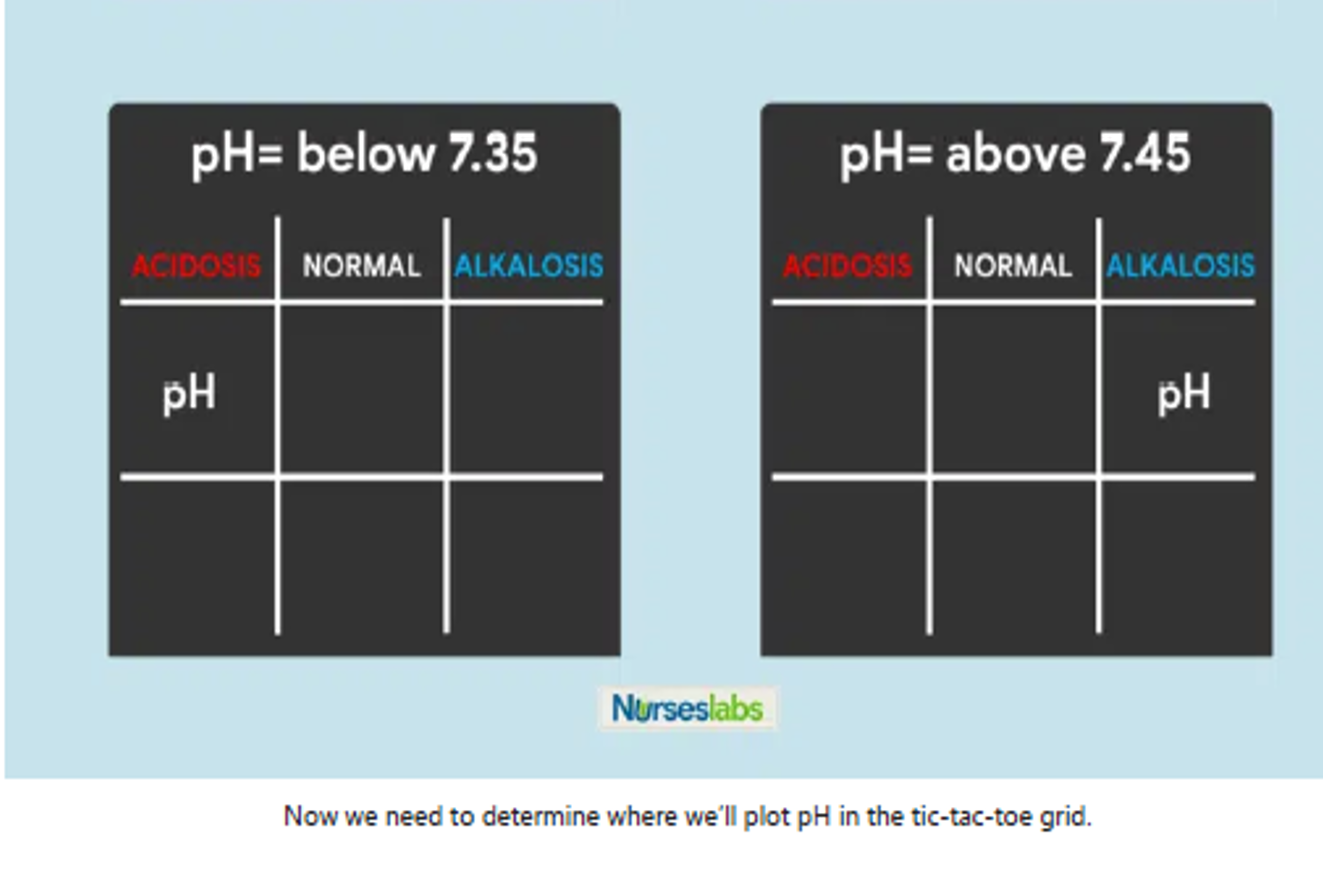
Determine if PaCO2 is Under Normal, Acidosis, or Alkalosis
- If PaCO2 is below 35, place it under the ALKALOSIS column.
- If PaCO2 is above 45, place it under the ACIDOSIS column.
- If PaCO2 is within its normal range, place it under the NORMAL column.
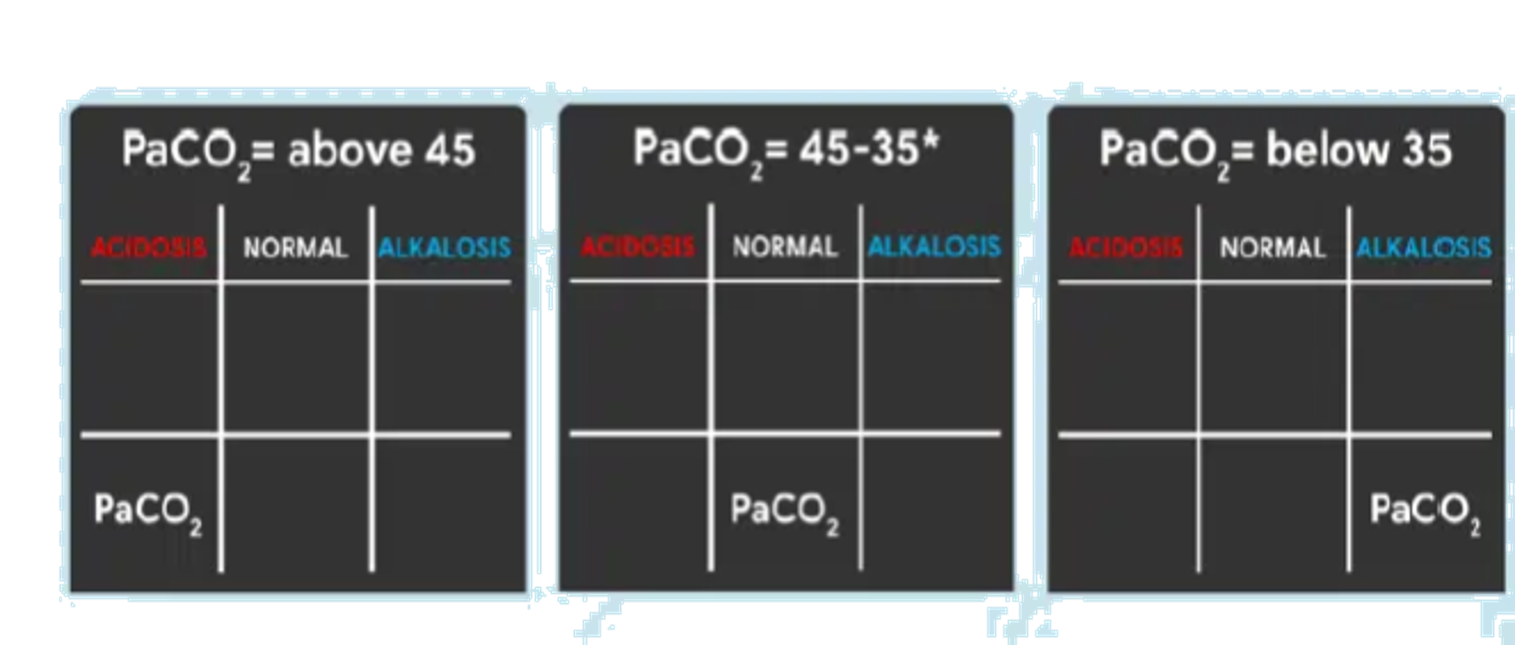
Determine if HCO3 is Under Normal, Acidosis, or Alkalosis
- If HCO3 is below 22, place it under the ACIDOSIS column.
- If HCO3 is above 26, place it under the ALKALOSIS column.
- If HCO3 is within its normal range, place it under the NORMAL column.
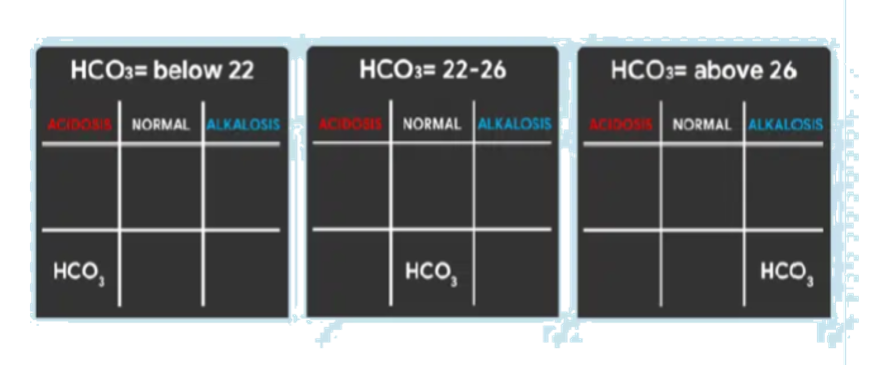
Determine if Values Define Metabolic or Respiratory
Looking back again on the tic-tac-toe grid, determine if pH is under the same column as PaCO2 or HCO3 so we can accomplish our goal of determining if the ABG is RESPIRATORY or METABOLIC.
- If pH is under the same column as PaCO2, it is RESPIRATORY.
- If pH is under the same column as HCO3, it is METABOLIC.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret accordingly.
Compensation
Determine the compensation:
- It is FULLY COMPENSATED if pH is normal.
- It is PARTIALLY COMPENSATED if all three (3) values are abnormal.
- It is UNCOMPENSATED if PaCO2 or HCO3 is normal and the other is abnormal.