Fluids, electrolytes, & Acid base disorders
Shaher Abbarah
Objectives
- Introduction
- Body fluids compartments
- Third space
- Functions of body fluids
- Fluids and electrolytes composition
- Fluids managements
- Acid base balance
Which of the following statements regarding total body water is False? A. In males, approximately 60% of total body weight is water B. The percentage of total body weight that is water is higher in males than in females C. The percentage of total body water decreases with age D. The majority of body water is contained within the interstitial fluid compartment Answer: D
Introduction
Total body water (TBW):
- 50-70 % total body weight
- Change with age, gender, & body size
- Higher in males, less in elderly
- Dec with age as muscle mass
- About 60%
Reasons of larger requirement of body fluids in infants, and children:
- Immature kidney: inability to concentrate
- Larger surface area per unit of body weight
- Greater metabolic activity
- 4 : 2 : 1 ratio
Introduction
- Amount required by babies: (approximate) at birth: about 75 mL/kg,
- First weeks age: 150 mL/kg.
- After the first month of life, use: 4:2:1
- Adults (70kg) about 35ml/kg
100:50:20 Rule - ADULT + Pedia, Per Day + Per hour 70 kg (10 x 100= 1000) + (10 x 50 = 500) + (50 x 20 = 1000) = 2500 p/d = 104 p/h
(10 x 100= 1000) + (10 x 50 = 500) + (70 x 20 = 1400) 2900 p/d
- 120ml p/h
4:2:1 more accurate for pediatrics 70 kg (10 x 4 | 10 x 2 | 50 x 1) 110/hr
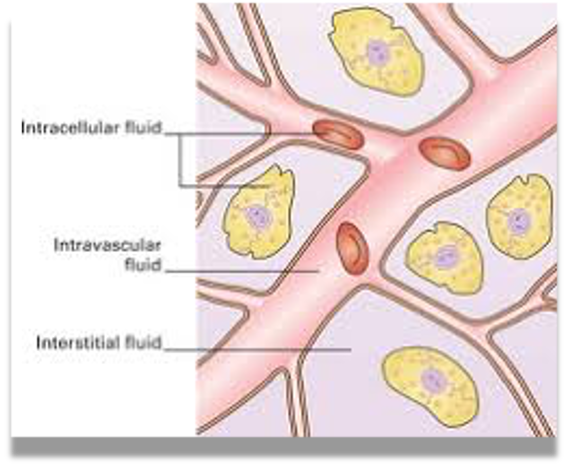
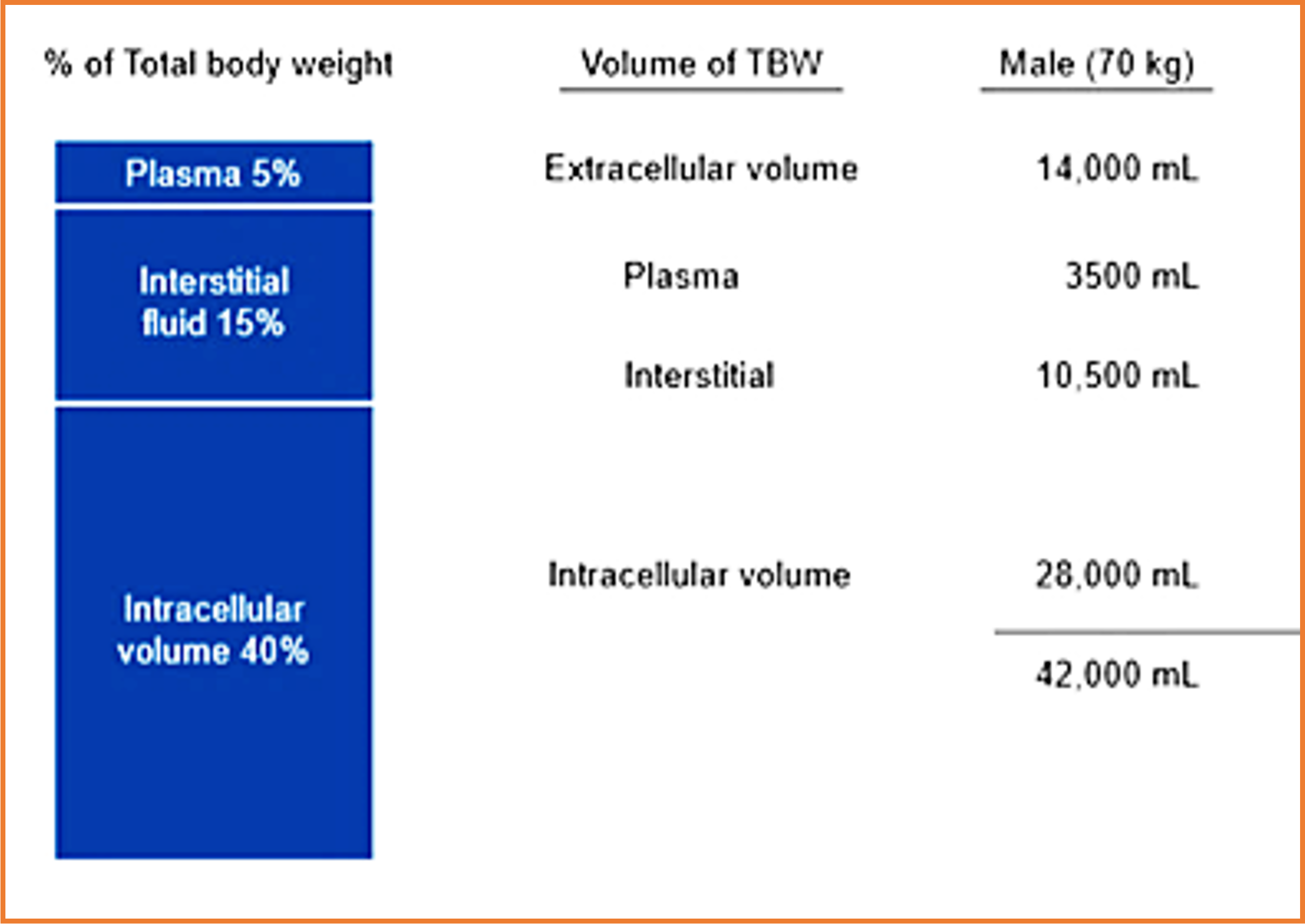
Body fluid compartments:
-
Intracellular fluid (ICF)
-
Extracellular fluid (ECF):
- Interstitial
- Intravascular
- Plasma ( 50 ml/kg body wt.)
- Blood ( 70ml/kg body wt)
Intracellular 40%, Extracellular 20% Interstitial: 15% Intravascular: 5%
Third space: Z
-
Abnormal transfer of extracellular body fluid from functional body fluid compartments to a non-functional compartments, either to body spaces, or in between cells.
-
No contribution to equilibrium
-
Unavailable as a reserve fluid
-
Unable to transport nutrients
-
Common locations:
- Tissue spaces (Edema)
- Abdomen (Ascites)
- Pleura (Effusion)
- Pericardium (Effusion)
-
Increased capillary permeability allows fluids, electrolytes, & proteins to leak from the vessels
- Causes:
- Injury or inflammation
- Massive trauma
- Crush injury
- Burns
- Sepsis - (Acute pancreatitis?)
- Cancer
- Bowel obstruction
Fluid accumulation in the interstitial space contributes to oedema Excess fluid moves from this space into the lymphatics (only up to a point) Edema is largely dependent on the net balance of hydrostatic and oncotic pressures inside the capillary and in the interstitium
Functions of body fluid:
- Medium for transport
- Cellular metabolism
- Solvent for electrolyte and other constituents
- Helps maintain body temperature
- Helps digestion and elimination
- Acts as lubricant
Body fluid turnover

- Minimum UOP in adult 30cc/hr (0.5 cc/kg/h)
- Fluid gain = Fluid loss
- Oxidative metabolism of protein & fat produce- 400-500 ml/ day
- Pyrexia increases water loss from the skin by approximately 200 mL/day for each 1°C rise in temperature
GI secretion composition
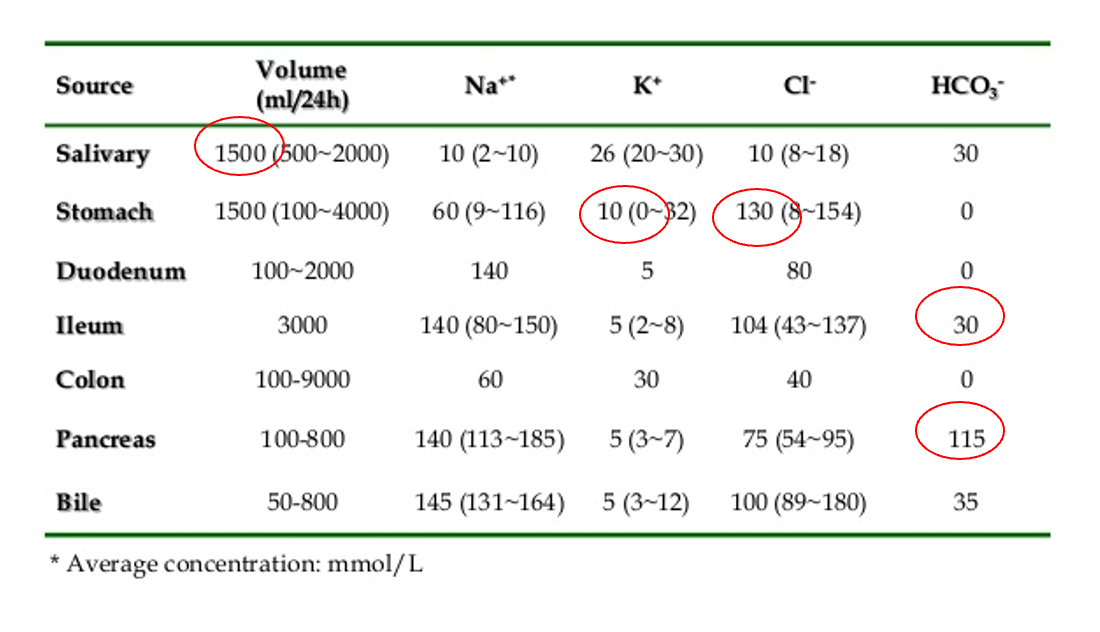
The metabolic derangement most commonly seen in patients with perfuse vomiting:
A. Hypochloremic , hypokalemic metabolic Alk B. Hypochloremic , hypokalemic metabolic Acid C. Hypochloremic, hyperkalemic metabolic Alk D. Hypochloremic, hyperkalemic metabolic Acid
Answer: A
non billous projectile vomitus pyloric stenosis pedia
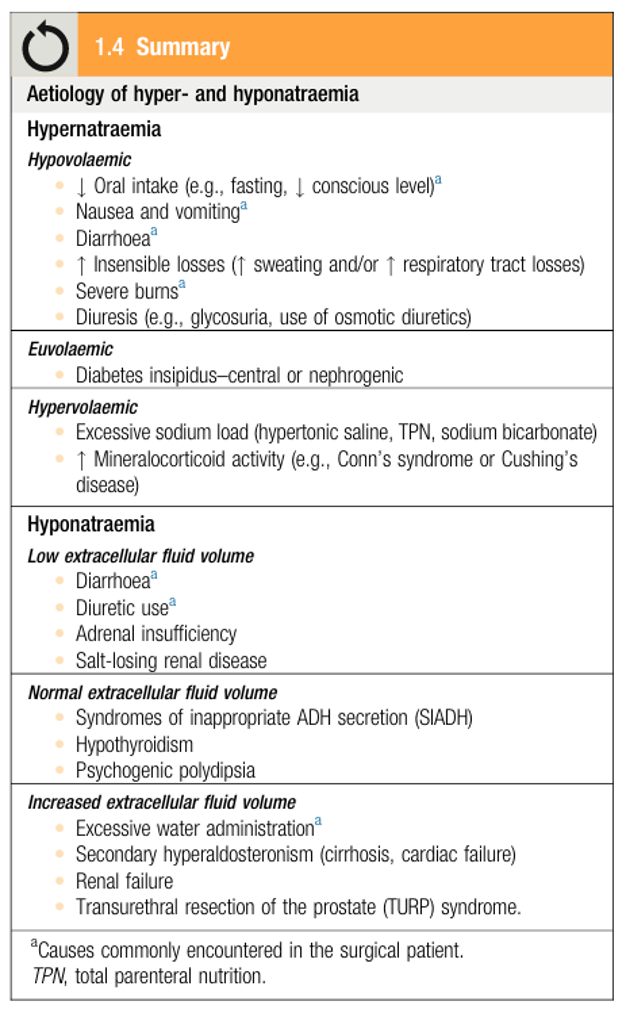
Electrolytes composition
Sodium (Na+): cataion
- Major determinant of body tonicity
- Primary extracellular cation
- Clinical manifestations are predominantly Neurologic.
- Avoid rapid correction of hyper/hyponatremia
Because …if chronic dont do anything? - differentiate with ACUTE with Hx - comatose, neuro
Z rapid correct hypo w/ sodium causing - central pontine demylenation -
correct hypern rapidly - cause brain edema
In hyponatremia it is first necessary to measure the serum osmolality to evaluate the tonicity imbalance.
In hypernatremia patients are categorized on the basis of their extracellular fluid volume status.
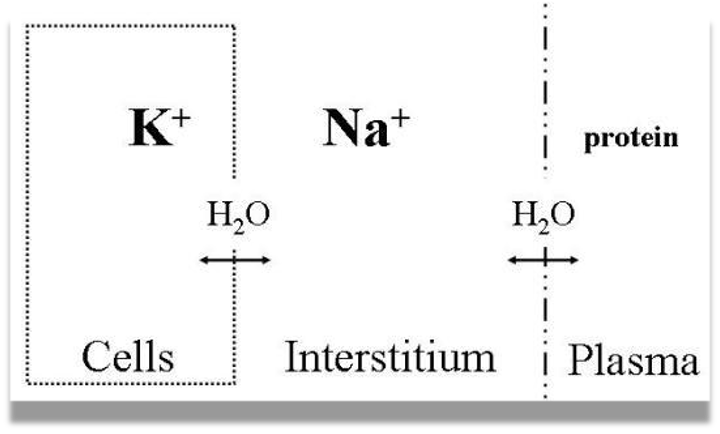
Electrolytes composition
- Intra-cellular Cations: “K” “Mg”, Anions: “Po” “protein”
- Extra-cellular Cation “Na” , Anions: “Cl” “Hco”
Potassium (K+): Z Read more about hyper/hypokalemia (P.15 in “Principle & Practice of Surgery”)
- Major intracellular cation
- 97% located in intracellular
- Compartment
- Tx of Hyperkalemia
Hyperkalemia - (Arrhythmias=Death) assure stable, potassium is really high, Hx, ECG, manage with membrane stabilizer shift (Insulin, B2 agonist), protect heart (calcium gluconate depends on guide), elimate K+ from body by ()
Hypokalemia Potassium chloride
 Hyponatremia, Hyporvolemic - Dilutational
TURP - Giving alot fluids
post op 2 hr developed seizure - approach?
assure vitally stable, maintain ABC - then investigate
Likely hyponatremia, osmolarity low
Hyponatremia, Hyporvolemic - Dilutational
TURP - Giving alot fluids
post op 2 hr developed seizure - approach?
assure vitally stable, maintain ABC - then investigate
Likely hyponatremia, osmolarity low
hypernatremia - High osmolarity losing only fluids - sodium is still in body - high osmolarity
- Severe Burn
Both Hypo HyperNa Diarrhea Fluids + Na or Fluids
Hyponatremia, Hypovolemia
Fluids,& electrolytes management
Before administering intravenous fluids the following should be considered:
- Fluid deficiency
- Fluid compartments requiring replacement
- Electrolyte imbalance
Read about “Assessing losses in the surgical patient” P. 11-12
Types of fluids:
- Isotonic, hypotonic, hypertonic
- IVF bolus :
- Given in a rapid manner,
- Isotonic fluids should used, to increase intravascular
- No Dextrose: to avoid hyperglycemia (except hypoglycemic attack)
- IVF maintenance
- Crystalloids vs colloids
Fluids,& electrolytes management
- Major determinants of osmotic activity in plasma are Na⁺, glucose & urea
- Normal serum osmolarity 285 mOsm/L
- Isotonic crystalloids are appropriate for correcting EFC losses
Fluids,& electrolytes management
- Dextrose added to IV fluid to inhibit muscle catabolism
- IVF bolus : IVF given in a rapid manner, isotonic fluids should used , to increase intravascular volume
 290-310 normal osmolarity
290-310 normal osmolarity
Isotonic - (0.9% ns) ORR LR ACC? CC
Hypotonic - 1/2 NS (0.45% ns)
Isotonic - LR - Ringer Lactate - motor accident / unstable - mimicking plasma content - (plasma potassium sodium lactate calcium) - lactate ⇒ bicarb ⇒ acidosis
Isotonic - D5W
Hypertonic - D5 1/2 NS - for maintenance - (preoperative postoperative) - glucose catabolism stimulate insulin for anabolic phase to participate - if normal saline body will turn into catabolism
- Hypertonic saline solutions have an osmolality greater than ECF and induce a shift of fluid from the IFC to the EFC
- Potential indications include:
- -treatment of cerebral oedema
- -raised intracranial pressure
- -hyponatraemic seizures
bullous as fast as you can for emergencies (hypoglycemia, loss conciousness) infusion - rate maintenence - slowly

- 3% NS - not used as infusion - used in hyponatremia - slow infusion to avoid demylenation
- D 50% - hypoglycemia
- Colloids (ICU)
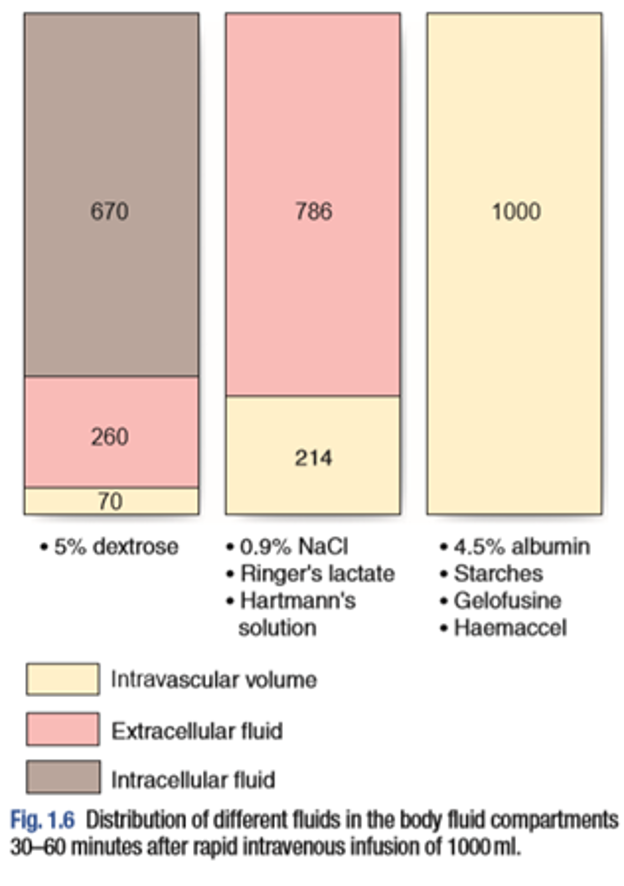
- Dextrose (glucose) - intracellular
- Ringer Lactate - Extracellular
- Albumins - Intravascular
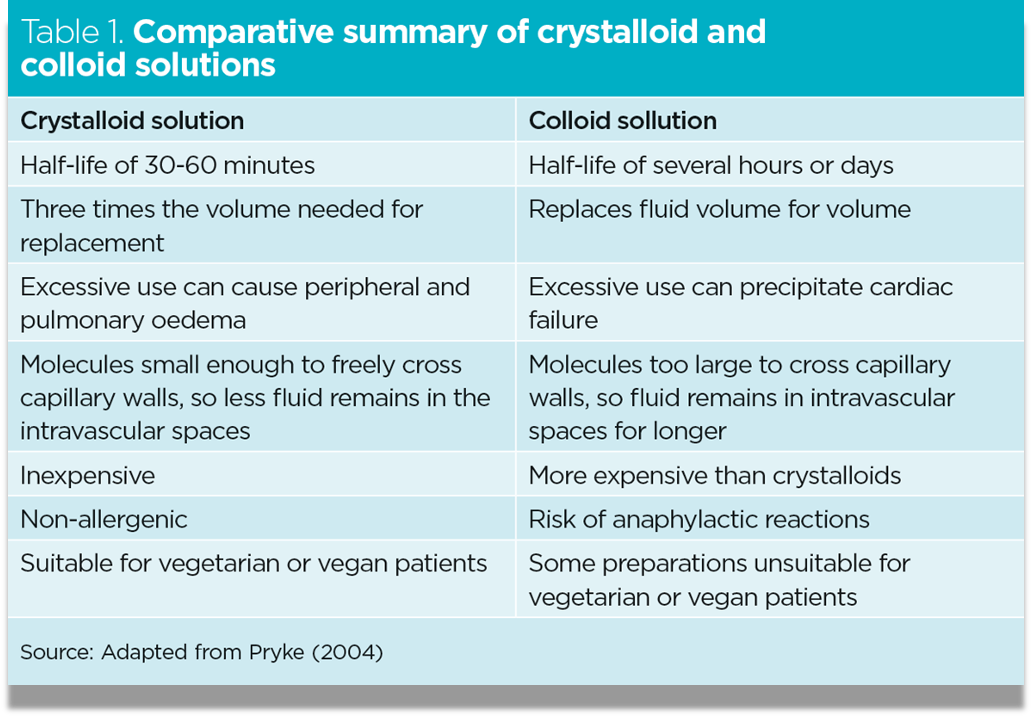 Colloids: Human Albumin , Dextran, Gelatin, HydroxyEthyl Starch
Colloids: Human Albumin , Dextran, Gelatin, HydroxyEthyl Starch
Side effects:
- -Coagulopathy
- -pruritus
- -anaphylaxis
- -Starch associated w/: Renal Failure, and mortality
Excessive administration of normal saline for fluid resuscitation can lead to what metabolic derangement? A. Metabolic Alk B. Metabolic Acid C. Respiratory Alk D. Respiratory Acid
Answer: B
Hyperchloremic metabolic acidosis
Aggressive fluid resuscitation with dextran (hetastarch) can result in: A/ Bleeding B/ Metabolic Acidosis C/ Seizures D/ renal failure
Medical conditions predispose to fluid and electrolytes abnormalities:
- Congestive heart failure
- Renal failure
- Cirrhosis
- GI loss
- Fistula/ stoma formation
Acid base balance
Acid Base Balance Most enzymatic reactions occur in a narrow PH range
3 primary systems to buffer PH:
-
Buffer system (Principally bicarbonate):
- Carbonate-Carbonate system in RBC
- Intracellular protein, phosphate, Hg, and minerals
- Henderson-Hasselbach equation (Bicarb H+ cal)
-
Respiratory system
- Eliminates CO2
-
Renal system
- Excretion of acid
- Reclamation of filtered bicarbonate
- Respiratory system is most Rapid (in minutes) ,, Then: Buffers (in hours) ,, Then: Renal (in days)
- Henderson-Hasselbalch: to determine the pH of a buffer by measuring the concentration of Acid and conjugated base.
- The Henderson-Hasselbalch equation allows for the calculation of pH from HCO3- and PCO2: pH = 6.1 + log([HCO3-]/[0.03 × pCO2])
- 6.1 = pKa of carbonic acid
- 0.03 = solubility constant of PCO2
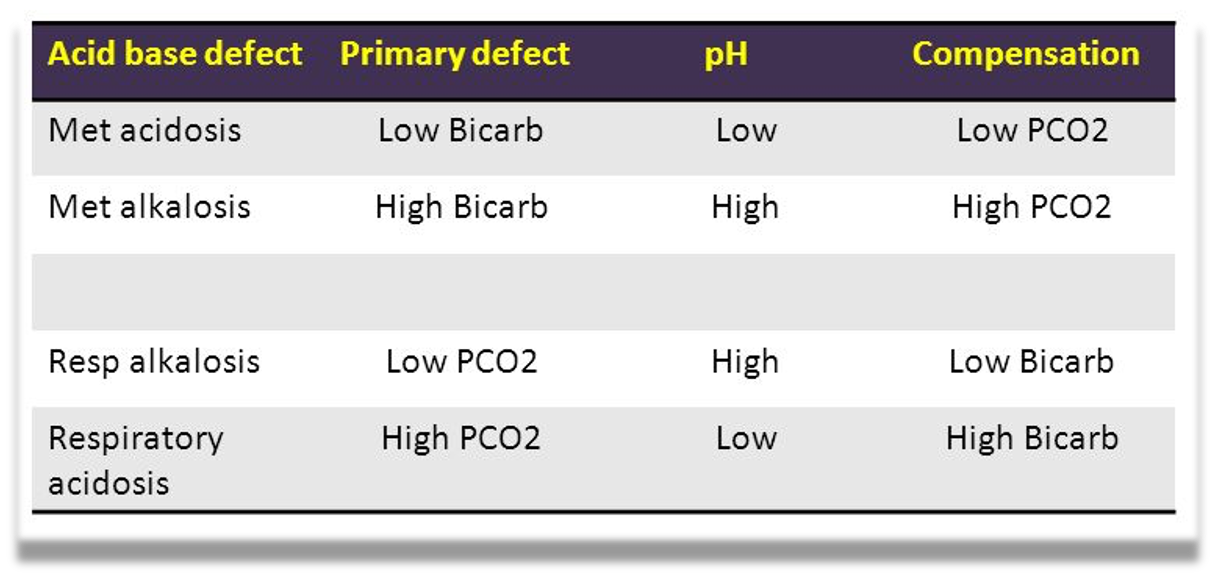
Compensatory mechanism:
Blood pH abnormalities
-
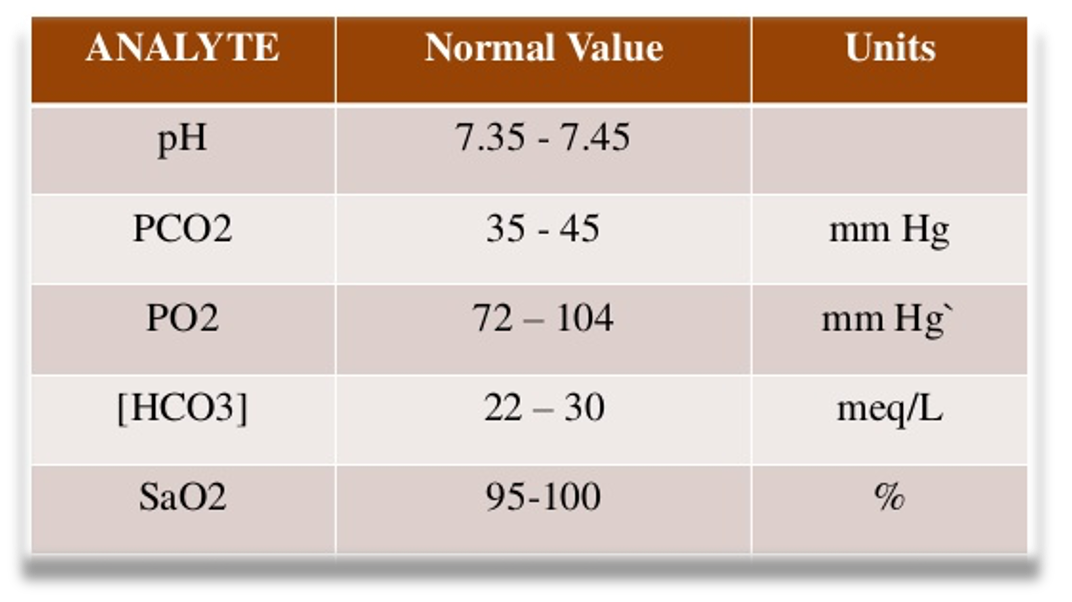
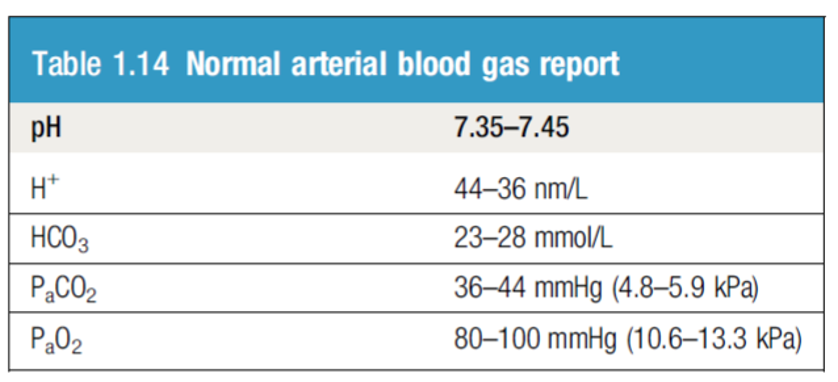
Acidemia: abnormally low blood pH (pH < 7.35)
-
Alkalemia: abnormally high blood pH (pH > 7.45)
-
Blood CO2: The normal range for arterial blood is 36–44 mm Hg.
-
Blood HCO3:
- The normal range is 23 – 28 mmol/L
Metabolic Acidosis
- increase in plasma hydrogen ions in conjunction with a decrease in bicarbonate concentration.
- Anion gap = Concentration of measured cations - Concentration of measured anions
- High Anion Gap Acidosis
- Normal Anion Gap Acidosis
- Read more about the Etiology of high / normal anion gap in metabolic acidosis “AMBOSS”
Anion Gap: (Na + K) – (HCO3 + Cl)
 **Patient newly diagnosed with Acute Kidney injury, Uremia
ABG showing:
PH 7.26
CO2 24
**Patient newly diagnosed with Acute Kidney injury, Uremia
ABG showing:
PH 7.26
CO2 24
HCO3 18
Metabolic Alkalosis
- Metabolic Alkalosis commonly associated with hypokalemia and hypochloremia.
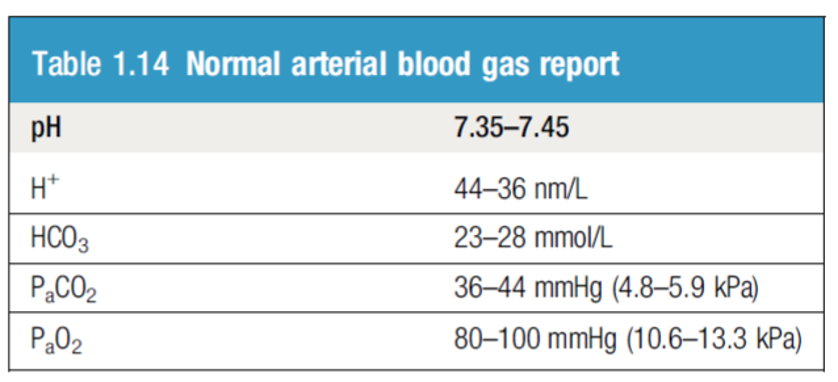 ICU patient, intubated on Mechanical Ventilation. Post OP, on NGT ABG showing:
PH 7.50
ICU patient, intubated on Mechanical Ventilation. Post OP, on NGT ABG showing:
PH 7.50
CO2 55
HCO3 35
Respiratory Acidosis
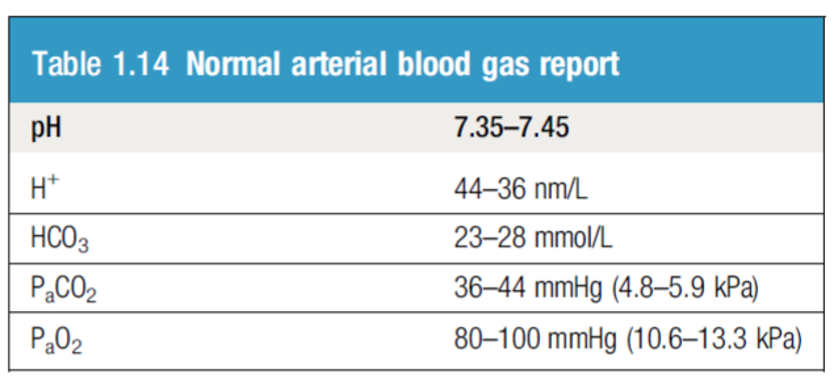 ICU patient, intubated on Mechanical Ventilation. low RR ABG showing:
PH 7.25
CO2 70
ICU patient, intubated on Mechanical Ventilation. low RR ABG showing:
PH 7.25
CO2 70
HCO3 35
- Causes: postoperative in hypoventilated patient excessive opiate administration
- In context of pulmonary complication (ex. Pneumonia)
Respiratory Alkalosis
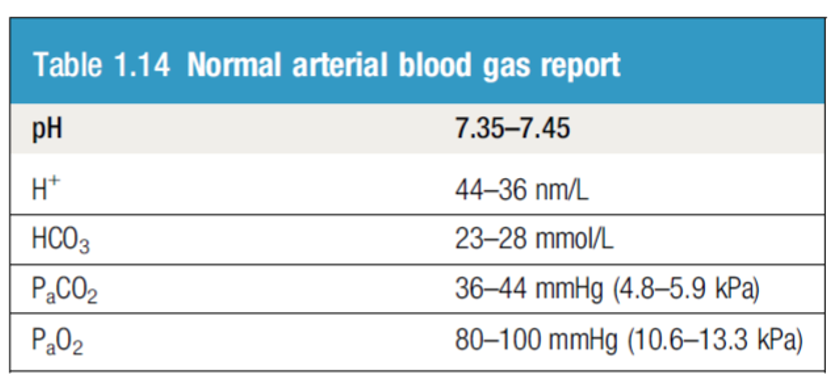 ICU patient, intubated on Mechanical Ventilation. Post OP, with high RR ABG showing:
PH 7.50
CO2 24
ICU patient, intubated on Mechanical Ventilation. Post OP, with high RR ABG showing:
PH 7.50
CO2 24
HCO3 18
Caused by excessive excretion of Co2 .. (Hyperventilation)