It is a gram-negative rod (coccobacillus). - A facultative anaerobe which grows best in media enriched with CO2.
Two major categories of H. influenzae were defined: the unencapsulated strains and the encapsulated strains.
-
Encapsulated strains were classified on the basis of their distinct capsular antigens.
- There are six generally recognized types of encapsulated H. influenzae: a, b, c, d, e, and f.
- Serotype b is the most virulent type.
- Organism found only in humans.
-
Unencapsulated strains are termed nontypable (NTHi) because they lack capsular serotypes.
- The unencapsulated strains are almost always less invasive.
The Incubation Period
It is not certain but could be as short as a few days.
Transmission
-
Haemophilus influenzae bacteria, including Hib, are spread person-to-person by direct contact or through respiratory droplets coughing and sneezing.
-
Usually, the bacteria remain in the nose and throat, causing no harm. Sometimes the bacteria can enter the blood and spread, causing serious infection in the individual.
Pathogenicity
- Enters the body through the respiratory tract;
- Two types of behaviors:
- Asymptomatic colonization
- Infections such as sinusitis, otitis media, or pneumonia.
- Two types of behaviors:
- Organism produces IgA protease which neutralizes respiratory mucosal IgA, this helps in its attachment to respiratory mucosa.
- After attachment to respiratory mucosa, it can enter the bloodstream and cause: Bacteremia and meningitis
- 95% of encapsulated forms (type b) are responsible for these diseases.
- Non-capsulated forms are responsible for otitis media, sinusitis, and pneumonia.
- In children, the age group 6 months - 6 years is most prone to infection by the organism.
- Peak incidence is from 6 months - 1 year.
- Virulence factors are polysaccharide capsule and endotoxin.
Risk Factors for Invasive Disease
Exposure factors:
- Household crowding
- Large household size
- Child care attendance
- Low socioeconomic status
- Low parental education
- School-aged siblings
Host factors:
- Race/ethnicity
- Chronic disease
Clinical Features
- Meningitis:
- Accounted for approximately 50%-65% of cases in the prevaccine era.
- Hearing impairment or neurologic sequelae in 15%-30%.
- Case-fatality rate 2%-5% despite effective antimicrobial therapy.
- Otitis media and sinusitis cause pain in affected areas and redness and bulging of the tympanic membrane.
- Septic arthritis, cellulitis, and sepsis (especially in splenectomized patients).
- Rarely epiglottitis in young children.
- Pneumonia in the elderly, especially those with chronic respiratory diseases.
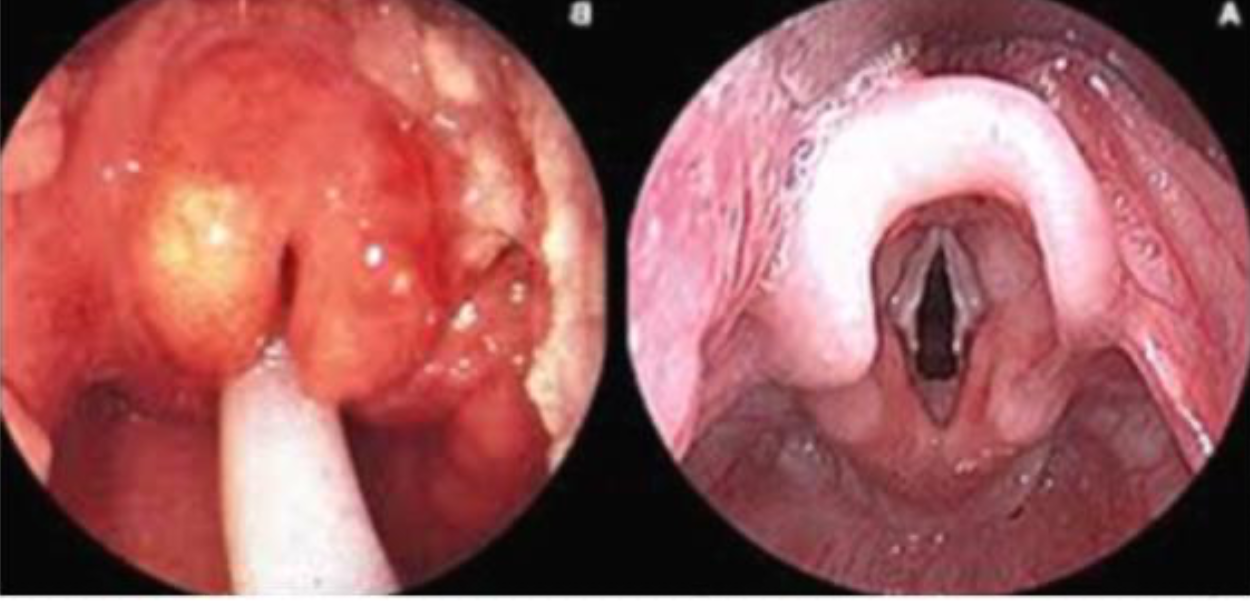
chart imageCC
Diagnosis
-
Diagnosis is considered confirmed when the organism is isolated from a sterile body site (cerebrospinal fluid or blood) which indicates H. influenzae infection.
-
In this respect, H. influenzae cultured from the nasopharyngeal cavity or sputum would not indicate H. influenzae disease (because these sites are colonized in disease-free individuals).
-
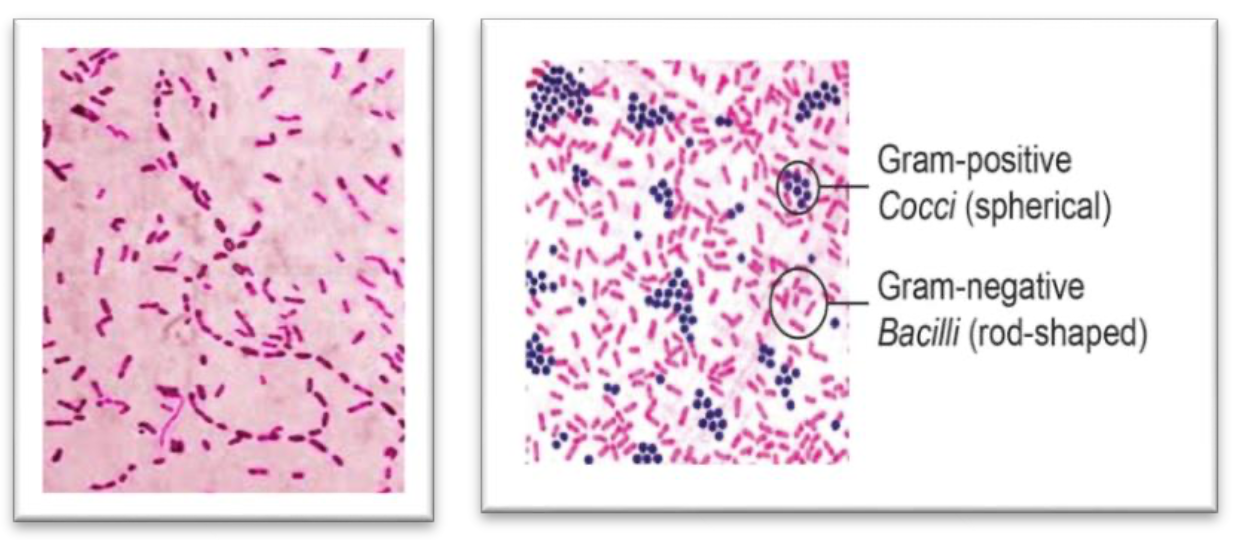
Gram staining.
-
Organism is grown on chocolate agar.
-
Definitive diagnosis can be made with Quellung test.
-
Additional means of identifying encapsulated strains include fluorescent-antibody staining of the organism and latex agglutination tests, which detect the capsular polysaccharide.
Treatment
- Ceftriaxone is the drug of choice in meningitis and other serious infections.
- Otitis media and sinusitis are treated with Augmentin.
Prevention
It is by vaccination. The vaccine given is called Hib. It is in conjugated form, conjugated with a carrier protein. Given between 2-15 months. Conjugated is more effective than the unconjugated one. Incidence has fallen 99% since the prevaccine era. Most recent cases reported are unvaccinated or incompletely vaccinated children.
Polysaccharide Conjugate Vaccines:
- Stimulates T-dependent immunity.
- Enhanced antibody production, especially in young children.
- Repeat doses elicit booster response.
Contraindications and Precautions:
- Severe allergic reaction to vaccine component or following a prior dose.
- Moderate or severe acute illness.
- Age less than 6 weeks.