FM
Treatment
Psychotherapy
Meta-analysis showed significantly higher remission rates among patients who received psychotherapy than among those in control conditions:
- Cognitive behavioral therapy [66%].
- Psychodynamic therapy [54%].
- Supportive counseling [49%].
- Behavioral activation [74%].
- Control conditions [43%].
Treatment
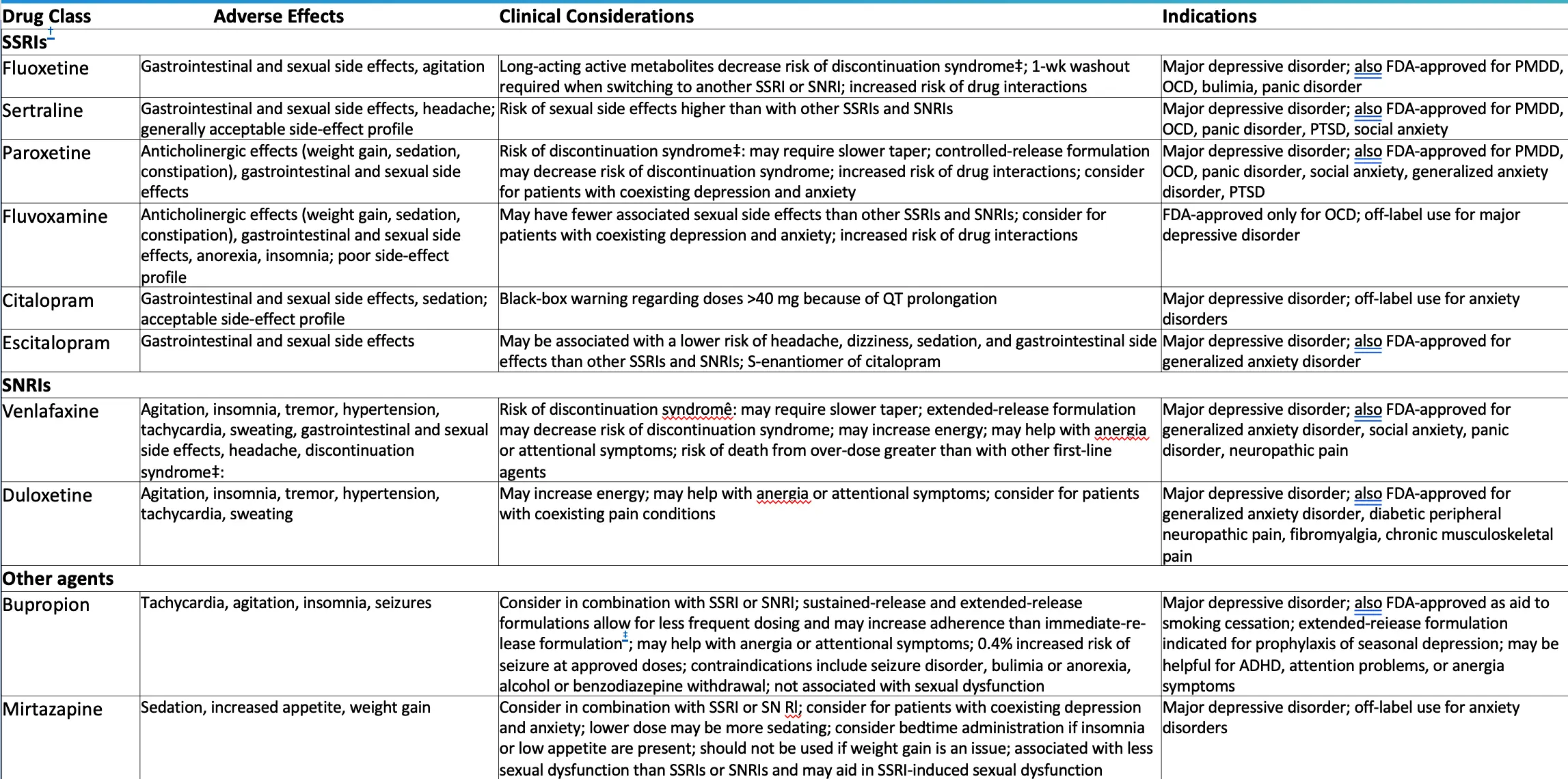
Drug Class
-
SSRIs
- Fluoxetine
- Adverse Effects: Gastrointestinal and sexual side effects, agitation
- Clinical Considerations: Long-acting active metabolites decrease risk of discontinuation syndrome‡; 1-wk washout required when switching to another SSRI or SNRI; increased risk of drug interactions
- Indications: Major depressive disorder; also FDA-approved for PMDD, OCD, bulimia, panic disorder
- Sertraline
- Adverse Effects: Gastrointestinal and sexual side effects, headache; generally acceptable side-effect profile
- Clinical Considerations: Risk of sexual side effects higher than with other SSRIs and SNRIs
- Indications: Major depressive disorder; also FDA-approved for PMDD, OCD, panic disorder, PTSD, social anxiety
- Paroxetine
- Adverse Effects: Anticholinergic effects (weight gain, sedation, constipation), gastrointestinal and sexual side effects
- Clinical Considerations: Risk of discontinuation syndrome‡; may require slower taper; controlled-release formulation may decrease risk of discontinuation syndrome; increased risk of drug interactions; consider for patients with coexisting depression and anxiety
- Indications: Major depressive disorder; also FDA-approved for PMDD, OCD, panic disorder, social anxiety, generalized anxiety disorder, PTSD
- Fluvoxamine
- Adverse Effects: Anticholinergic effects (weight gain, sedation, constipation), gastrointestinal and sexual side effects, anorexia, insomnia; poor side-effect profile
- Clinical Considerations: May have fewer associated sexual side effects than other SSRIs and SNRIs; consider for patients with coexisting depression and anxiety; increased risk of drug interactions
- Indications: FDA-approved only for OCD; off-label use for major depressive disorder
- Citalopram
- Adverse Effects: Gastrointestinal and sexual side effects, sedation; acceptable side-effect profile
- Clinical Considerations: Black-box warning regarding doses >40 mg because of QT prolongation
- Indications: Major depressive disorder; off-label use for anxiety disorders
- Escitalopram
- Adverse Effects: Gastrointestinal and sexual side effects
- Clinical Considerations: May be associated with a lower risk of headache, dizziness, sedation, and gastrointestinal side effects than other SSRIs and SNRIs; S-enantiomer of citalopram
- Indications: Major depressive disorder; also FDA-approved for generalized anxiety disorder
- Fluoxetine
-
SNRIs
- Venlafaxine
- Adverse Effects: Agitation, insomnia, tremor, hypertension, tachycardia, sweating, gastrointestinal and sexual side effects, headache, discontinuation syndrome‡
- Clinical Considerations: Risk of discontinuation syndrome‡; may require slower taper; extended-release formulation may decrease risk of discontinuation syndrome; may increase energy; may help with anergia or attentional symptoms; risk of death from overdose greater than with other first-line agents
- Indications: Major depressive disorder; also FDA-approved for generalized anxiety disorder, social anxiety, panic disorder, neuropathic pain
- Duloxetine
- Adverse Effects: Agitation, insomnia, tremor, hypertension, tachycardia, sweating
- Clinical Considerations: May increase energy; may help with anergia or attentional symptoms; consider for patients with coexisting pain conditions
- Indications: Major depressive disorder; also FDA-approved for generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, chronic musculoskeletal pain
- Venlafaxine
-
Other agents
- Bupropion
- Adverse Effects: Tachycardia, agitation, insomnia, seizures
- Clinical Considerations: Consider in combination with SSRI or SNRI; sustained-release and extended-release formulations allow for less frequent dosing and may increase adherence than immediate-release formulation*; may help with anergia or attentional symptoms; 0.4% increased risk of seizure at approved doses; contraindications include seizure disorder, bulimia or anorexia, alcohol or benzodiazepine withdrawal; not associated with sexual dysfunction
- Indications: Major depressive disorder; also FDA-approved as aid to smoking cessation; extended-release formulation indicated for prophylaxis of seasonal depression; may be helpful for ADHD, attention problems, or anergia symptoms
- Mirtazapine
- Adverse Effects: Sedation, increased appetite, weight gain
- Clinical Considerations: Consider in combination with SSRI or SNRI; consider for patients with coexisting depression and anxiety; lower dose may be more sedating; consider bedtime administration if insomnia or low appetite are present; should not be used if weight gain is an issue; associated with less sexual dysfunction than SSRIs or SNRIs and may aid in SSRI-induced sexual dysfunction
- Indications: Major depressive disorder; off-label use for anxiety disorders
- Bupropion
When to refer?
- If the diagnosis is doubtful
- If there is a risk for suicide
- If drug and alcohol dependence or withdrawal complicate the management
- If psychosis appears to be involved
- Failure of response to basic treatment
- Hospitalization is indicated
- According to the secondary causes
Psychiatry
Depression Management
Pharmacotherapy:
- 50 to 70% effective
Selection factors:
- ➢ Prior response to agent
- ➢ Anticipated side effects
- ➢ Concomitant illness
- ➢ Potential for drug interactions
- ➢ Family history of response
- ➢ Patient desire
- ➢ Cost
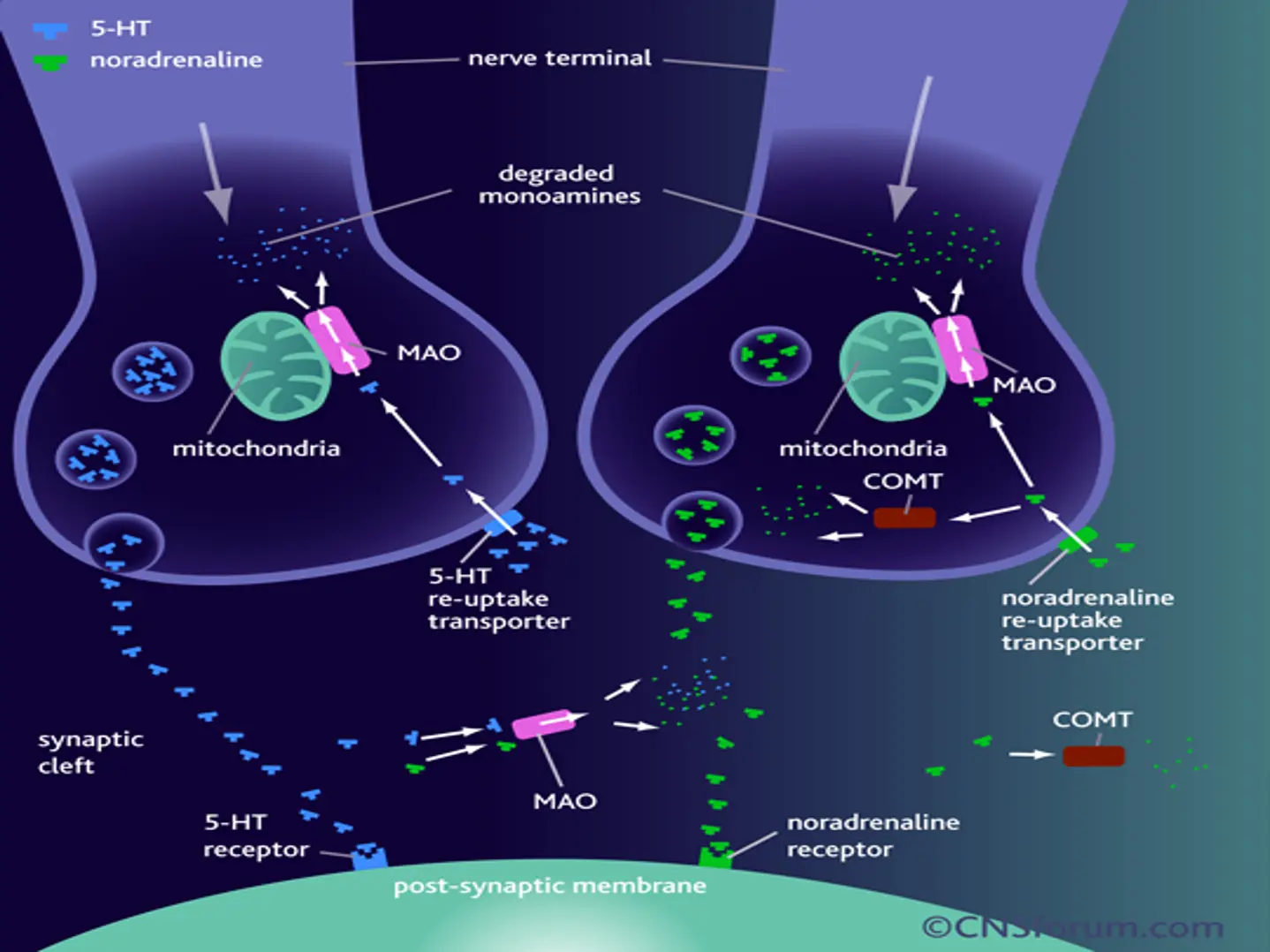
Serotonin and SSRIs
- A class of drugs that inhibit the uptake of serotonin so increases synaptic serotonin levels.
- Many SSRIs affect other receptors especially at higher doses.
- Several serotonin (5-HT) receptors subtypes.
- The different response we notice in some patients with one antidepressant versus another because they are structurally unrelated and different response related to different morphology of serotonin transport protein.
Middleton et al: BMJ (2005)
SSRIs
- Fluoxetine : 1988
- Sertraline : 1992
- Paroxetine : 1993
- Fluvoxamine : 1994
- Citalopram : 1998
- S. Citalopram : 2002
SNRIs
- Desvenlafaxine, duloxetine, venlafaxine
Norepinephrine-Serotonin Modulator
NASA
- Mirtazapine
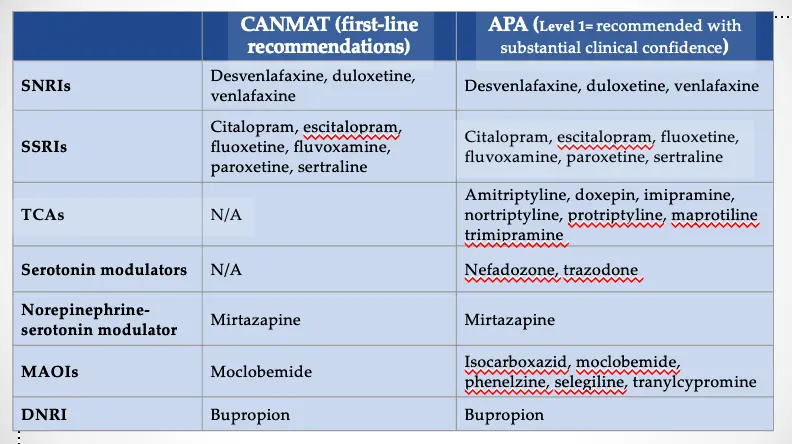
Antidepressants: Guidelines Y
-
CANMAT (first-line recommendations)
- SNRIs
- Desvenlafaxine, duloxetine, venlafaxine
- SSRIs
- Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline
- TCAs
- N/A
- Serotonin modulators
- N/A
- Norepinephrine-serotonin modulator
- Mirtazapine
- MAOIs
- Moclobemide
- DNRI
- Bupropion
- SNRIs
-
APA (Level 1= recommended with substantial clinical confidence)
- SNRIs
- Desvenlafaxine, duloxetine, venlafaxine
- SSRIs
- Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline
- TCAs
- Amitriptyline, doxepin, imipramine, nortriptyline, protriptyline, maprotiline, trimipramine
- Serotonin modulators
- Nefazodone, trazodone
- Norepinephrine-serotonin modulator
- Mirtazapine
- MAOIs
- Isocarboxazid, moclobemide, phenelzine, selegiline, tranylcypromine
- DNRI
- Bupropion
- SNRIs
APA = American Psychiatric Association; CANMAT = Canadian Network for Mood and Anxiety Treatments; TCA = tricyclic antidepressant; N/A = not applicable; MAOI = monoamine oxidase inhibitor; DNRI = dopamine norepinephrine reuptake inhibitor
Gelenberg et al., 2010. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. Third Edition. Available at APA Guidelines; Lam RW et al. J Affect Disord. 2009;117(Suppl 1):S26-S43.
Principles of Prescribing in Depression
- Discuss with the patient choice of drug and utility/availability of other, non-pharmacological treatments.
- Discuss with the patient likely outcomes, such as gradual relief from depressive symptoms over several weeks.
- Prescribe a dose of antidepressant (after titration, if necessary) that is likely to be effective.
- For a single episode, continue treatment for at least 6-9 months after resolution of symptoms (multiple episodes may require longer).
- Withdraw antidepressants gradually; always inform patients of the risk and nature of discontinuation symptoms.
Summary of NICE Guidance: Treatment for Depression
- Antidepressants are not recommended as a first-line treatment in recent-onset, mild depression; active monitoring, individual guided self-help, CBT and/or exercise are preferred.
- Antidepressants are recommended for the treatment of moderate-to-severe depression and for dysthymia.
- When an antidepressant is prescribed, a selective serotonin reuptake inhibitor (SSRI) is recommended.
- All patients should be informed about the withdrawal (discontinuation) effects of antidepressants.
- For treatment-resistant depression, recommended strategies include: Z
- Augmentation with lithium or an antipsychotic
- The addition of a second antidepressant.
- Patients with two prior episodes and functional impairment should be treated for at least 2 years.
- The use of ECT is supported in severe and treatment-resistant depression.
Selective Serotonin Reuptake Inhibitors: Summary of Suggested Treatment
- Use of the lowest possible dose
- Monitor closely in early stages for restlessness, agitation and suicidality. This is particularly important in young people (<30 years).
- Doses should be tapered gradually on stopping.
Key Points That Patients Should Know About Depression
- A single episode of depression should be treated for at least 6-9 months after remission.
- The risk of recurrence of depressive illness is high and increases with each episode.
- Those who have had multiple episodes may require treatment for many years.
- The chances of staying well are greatly increased by taking antidepressants.
- Antidepressants are:
- Effective
- Not addictive
- Not known to lose their efficacy over time
- Not known to cause new long-term side effects.
- Medications need to be continued at the treatment dose. If side effects are intolerable, it may be possible to find a more suitable alternative.
- If patients decide to stop their medication, this must not be done abruptly, as this may lead to unpleasant discontinuation effects and confers a higher risk of relapse. The medication needs to be reduced slowly under the supervision of a doctor.
The World Health Organisation figures from 1997 show that the number of reported deaths from suicide is high compared to more high-profile causes of death. The actual figure is likely to be much higher as many suicides are not reported. According to the WHO figures for some countries, such as Mexico, do not even report death by suicides. This indicates a discrepancy in the way mortality figures are reported.